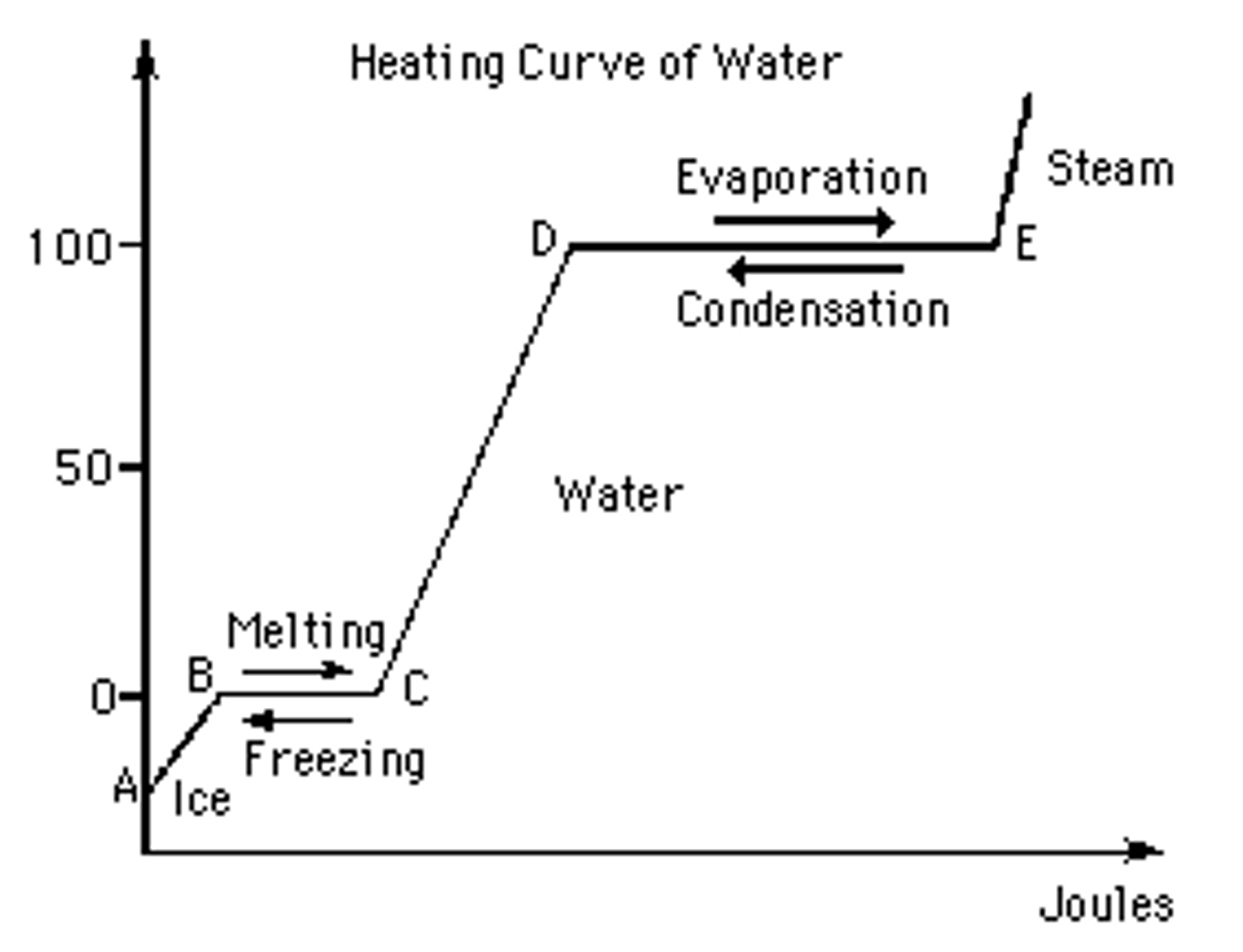
Heating Curve Liquid State . All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: heating curves show how the temperature changes as a substance is heated up. A superheated liquid, a liquid at a. heating curves relate temperature changes to phase transitions. heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. summary of state changes. Cooling curves are the opposite. Changes from one state to. They show how the temperature changes.
from www.chegg.com
this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: A superheated liquid, a liquid at a. They show how the temperature changes. All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. heating curves relate temperature changes to phase transitions. heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. summary of state changes. Changes from one state to.
Solved The graph above shows the heating curve of water. One
Heating Curve Liquid State this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. Changes from one state to. summary of state changes. heating curves relate temperature changes to phase transitions. All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. They show how the temperature changes. Cooling curves are the opposite. A superheated liquid, a liquid at a. heating curves show how the temperature changes as a substance is heated up. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate:
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Heating Curve Liquid State A superheated liquid, a liquid at a. summary of state changes. heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. heating curves relate temperature changes to phase transitions. heating curves show how the temperature changes as a substance is heated. Heating Curve Liquid State.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Liquid State heating curves show how the temperature changes as a substance is heated up. heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. Heating Curve Liquid State.
From www.excelatphysics.com
Heating Curve ExcelPhysics Heating Curve Liquid State Cooling curves are the opposite. A superheated liquid, a liquid at a. heating curves relate temperature changes to phase transitions. Changes from one state to. They show how the temperature changes. All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. heating curve a common form. Heating Curve Liquid State.
From chem-net.blogspot.com
Phase Changes Energy Changes Heating Curves Chemistry Net Heating Curve Liquid State heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. Changes from one state to. summary of state changes. heating curves relate temperature changes to phase transitions. this plot of temperature shows what happens to a 75 g sample of ice. Heating Curve Liquid State.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Pdf Heating Curve Liquid State heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: summary of state. Heating Curve Liquid State.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes Heating Curve Liquid State heating curves show how the temperature changes as a substance is heated up. heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. Cooling curves are the opposite. A superheated liquid, a liquid at a. They show how the temperature changes. All of. Heating Curve Liquid State.
From chem.libretexts.org
3.7.0.0 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Heating Curve Liquid State All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. They show how the temperature changes. heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. Changes from one state to. Cooling. Heating Curve Liquid State.
From webmis.highland.cc.il.us
Changes of State Heating Curve Liquid State Changes from one state to. heating curves show how the temperature changes as a substance is heated up. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: A superheated liquid, a liquid at a. heating curves relate. Heating Curve Liquid State.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation Heating Curve Liquid State A superheated liquid, a liquid at a. summary of state changes. heating curves relate temperature changes to phase transitions. heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. They show how the temperature changes. All of the changes of state that occur between solid, liquid and gas are. Heating Curve Liquid State.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Liquid State summary of state changes. Changes from one state to. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: heating curve a common form of depicting the temperature at which a substance changes states and also how much. Heating Curve Liquid State.
From chem.libretexts.org
10.3 Phase Transitions Chemistry LibreTexts Heating Curve Liquid State heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: Changes from one state. Heating Curve Liquid State.
From getrevising.co.uk
States of Matter Revision Cards in IGCSE Chemistry Heating Curve Liquid State summary of state changes. All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: heating curves. Heating Curve Liquid State.
From www.pinterest.com
Heating curve calculation (benzene) Worksheets, Printable preschool Heating Curve Liquid State They show how the temperature changes. A superheated liquid, a liquid at a. summary of state changes. heating curves relate temperature changes to phase transitions. Changes from one state to. heating curves show how the temperature changes as a substance is heated up. All of the changes of state that occur between solid, liquid and gas are. Heating Curve Liquid State.
From philschatz.com
Phase Change and Latent Heat · Physics Heating Curve Liquid State All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. heating curves relate temperature changes to phase transitions. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant. Heating Curve Liquid State.
From kittyx-tomow.blogspot.com
Heating Curve Of Water Heating Curve Of Water Heating curve basics Heating Curve Liquid State summary of state changes. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c as heat is added at a constant rate: They show how the temperature changes. Changes from one state to. Cooling curves are the opposite. All of the changes of state that occur between. Heating Curve Liquid State.
From askfilo.com
The graph below shows the heating curve for a pure substance. The tempera.. Heating Curve Liquid State heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. Cooling curves are the opposite. They show how the temperature changes. All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. Changes. Heating Curve Liquid State.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Liquid State They show how the temperature changes. Changes from one state to. heating curve a common form of depicting the temperature at which a substance changes states and also how much heat is required to change state. All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. . Heating Curve Liquid State.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Liquid State heating curves relate temperature changes to phase transitions. All of the changes of state that occur between solid, liquid and gas are summarized in the diagram in the figure below. A superheated liquid, a liquid at a. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23 c. Heating Curve Liquid State.